Authors:
(1) Renato P. dos Santos, CIAGE – Centre for Generative Artificial Intelligence in Cognition and Education.
Table of Links
Conceptualizing chemical reactions
Deepening on understanding of chemical reactions
Question about a graph of gases turning into water over time
Question about the difference between atoms, molecules, and moles
Deepening on the concept of mole
Question about changing of state
Question about an animated representation of water molecules undergoing phase changes
Question about plasma, a state of matter
Question about chemical bondings
Question about illustration of chemical bonds
Question about the essence of the type of chemical bonding
Limitations of the study and possible future studies
Author Contributions, Conflicts of interest, Acknowledgements, and References
Conceptualizing chemical reactions
P1: I have difficulty with this business of chemical reactions and balance
ChatGPT: Certainly! Balancing chemical reactions is a fundamental concept in chemistry. Let's break it down step by step.
Imagine you have a simple equation:
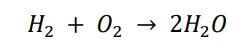
At first glance, it might seem like everything is in order. Hydrogen and oxygen react to produce water. But if we look more closely, there's a discrepancy in the number of atoms on both sides. Would you like to take a stab at identifying where the imbalance lies in this equation?
BingChat: I understand that balancing chemical equations can be challenging, but it is also very important for understanding how chemical reactions work. Balancing chemical equations means making sure that the number and type of atoms on the reactant side (left) are equal to the number and type of atoms on the product side (right). This is based on the law of conservation of mass, which states that matter cannot be created or destroyed in a chemical reaction[1].
To balance a chemical equation, you need to follow some steps:
• Identify the reactants and products and write their chemical formulas.
• Count the number of atoms of each element on both sides of the equation.
• Use coefficients (numbers in front of the formulas) to adjust the number of atoms of each element until they are equal on both sides. Do not change the subscripts (numbers after the symbols) as they indicate the composition of the compounds.
• Start with the element that appears only once on each side, and then move on to the other elements. Try to balance the oxygen and hydrogen atoms last, as they are often present in more than one compound.
• Check your work by counting the atoms again and making sure they are equal on both sides.
Here is an example of how to balance a simple chemical equation:
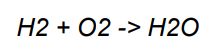
• The reactants are hydrogen gas (H2) and oxygen gas (O2), and the product is water (H2O).
• The number of hydrogen atoms on the left is 2, and on the right is 2. The number of oxygen atoms on the left is 2, and on the right is 1.
• To balance the oxygen atoms, we need to put a coefficient of 2 in front of H2O, so that we have 2 oxygen atoms on both sides. This also changes the number of hydrogen atoms on the right to 4.
• To balance the hydrogen atoms, we need to put a coefficient of 2 in front of H2, so that we have 4 hydrogen atoms on both sides. This does not affect the number of oxygen atoms, as they are already balanced.
• The final balanced equation is:
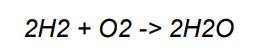
• We can check our work by counting the atoms again: 4 hydrogen and 2 oxygen on both sides.
I hope this explanation helps you understand how to balance chemical equations. If you want to practice more, you can use some online tools that can help you balance equations and check your answers. For example, you can use this online balancer[1], this interactive simulation[2], or this video tutorial[3]
Bard*: Balancing chemical reactions can be difficult at first, but it is an essential skill for any chemistry student. The key to balancing chemical reactions is to understand the law of conservation of mass, which states that matter cannot be created or destroyed. This means that the total number of atoms of each element on the left side of a chemical equation must be equal to the total number of atoms of that element on the right side of the equation. Here are some tips for balancing chemical reactions:*
Identify the reactants and products. The reactants are the substances that participate in the chemical reaction, and the products are the substances that are formed as a result of the reaction.
Write down the chemical formulas for all of the reactants and products. Be sure to include the state of each substance (solid, liquid, gas).
Count the number of atoms of each element on both sides of the equation.
Add coefficients to the reactants and products to make the number of atoms of each element equal on both sides of the equation. Coefficients are small numbers that are placed in front of the chemical formulas.
Here is an example of how to balance a chemical equation:
Unbalanced equation:
CH4 + O2 → CO2 + H2O
Balanced equation:
CH4 + 2O2 → CO2 + 2H2O
To balance this equation, we need to add a coefficient of 2 in front of the O2 on the left side of the equation. This will make the number of oxygen atoms equal on both sides of the equation. Once you have balanced a chemical equation, you can be sure that it represents a valid chemical reaction.
Here are some additional tips for balancing chemical reactions:
Use the smallest possible coefficients. This will make the equation easier to read and understand.
If you are having trouble balancing an equation, try breaking it down into smaller, simpler equations.
There are many online resources and tools that can help you to balance chemical equations. If you are still having difficulty with balancing chemical reactions, please feel free to ask me for help. I am always happy to assist students with their chemistry homework.
Claude*: I understand feeling confused about balancing chemical equations. It can seem complicated at first, but with some practice it becomes more intuitive. Let's break it down stepby-step:*
The first thing to understand is that during a chemical reaction, atoms are neither created nor destroyed - they just get rearranged into new configurations. This is known as the law of conservation of mass.
So when you balance an equation, you are making sure both sides have the same number and type of atoms. For example, let's look at the reaction between hydrogen and oxygen to form water:
H2 + O2 → H2O
Right now this equation is not balanced, because there are 2 hydrogen atoms on the left but only 1 on the right. To fix it, we need to add coefficient numbers in front to balance the atoms:
2H2 + O2 → 2H2O
Now there are 2 hydrogen atoms on both sides. We also balanced the oxygen by having 2 oxygen molecules on the left and right.
The key is to take it step-by-step and make sure you account for each element properly. I suggest starting with the more complex molecules first. Also, don't be afraid to use fractions or multiples if needed.
Does this help explain the basic logic behind balancing equations? Let me know if you need any clarification or want to walk through another example!
ChatGPT: shines in the Engagement & Interactivity category by directly involving the student by asking, "Would you like to take a stab at identifying where the imbalance lies in this equation?". The real-world example of a water molecule formation resonates with the Use of Analogy & Comparative Illustrations category. There's also a sense of Reinforcement & Feedback when it breaks down the reaction and points out the discrepancy. BingChat, on the other hand, demonstrates robust Instructional Strategies, walking the student through a step-by-step process. Its detailed explanation of balancing equations, specifically focusing on the process and techniques, speaks to Detail Depth & Content Recommendation. Offering online tools for further learning also fits into this category. The inclusion of emoticons brings in the Personalization & Personable Touch aspect. Bard’s response starts by validating the student's feelings, indicating a hint of Personalization & Personable Touch and uses the Use of Analogy & Comparative Illustrations category with relevant examples. Finally, it embodies Reinforcement & Feedback by offering additional tips on balancing reactions and an invitation for more questions point towards. Claude leans heavily into Instructional Strategies with a detailed step-by-step breakdown and an emphasis on the law of conservation of mass to explain the logic behind the balancing and into Engagement & Interactivity and Reinforcement & Feedback through an invitation for further questions.
This paper is available on arxiv under CC BY-SA 4.0 DEED license.